In a recent a blog post (nature vs. nurture), I outlined how it is that we aren’t much closer to understanding what makes each of the clones of Orphan Black totally unique. Though they sure do look alike, Orphan Black Wikia - Sarah Manning (Tatiana Maslany) is a rebel, Orphan Black Wikia - Alison Hendrix (Tatiana Maslany) is a literal soccer mom, and Helena (obviously, Tatiana Maslany) is a seemingly psychopathic assassin.
What do we know so far? (1) Other than short, clone-specific synthetic sequences, Sarah and her sisters share a nearly identical genome. (2) All of the clones we’ve met so far have been raised under totally different conditions. (However, it appears that the uniformly militaristic male clones—from Project Leda’s sibling, Project Castor—have all been reared together, which may explain why they are all been cold-blooded killers with a “glitch” that eventually leaves their brains looking like Swiss cheese.) In that previous blog post, I also summarized twin studies, the closest thing we have in the real word to clone studies, and I argued that the early experiences of the sestras, which range from the mundane to the insane, are what has made them such divergent personalities.
However, I did briefly suggest another possible explanation for their diversity—a phenomenon known as epigenetics. The topic of nature vs. nurture was enough from most people to digest in one blog post so I put off epigenetics for another day. (Regular readers of this series, specifically my analysis of “the spindle protein” problem, may also remember that epigenetics is a central focus of cloning research.) So let’s explore the subject in the depth it deserves to determine if epigenetics can explain the differences among Sarah, Cosima, Beth (RIP), Alison, Tony, Katja (also RIP), Helena, and the rest.
First, I must offer a general warning. Epigenetics has been explored relatively little. Cutting-edge science doesn’t resemble the tidy, logical explanations in text books. Currently, biologists are very excited about epigenetics, but by the same token, no one understands its full impact. The term epigenetics has been around for most of a century, but the contemporary meaning has only solidified within the last 20 years.
“Epi” is Greek for “on top of” so epigenetics literally means “on top of genetics.” But what does that mean? Generally, I try to avoid jargon, but a proper explanation of epigenetics requires an understanding of the Central Dogma of Molecular Biology, which summarizes the process by which genes encoded in DNA are transcribed into RNA strands, which are translated into proteins, which partially underlie all visible phenotypes. (If these terms have you utterly lost, please check out the first article in this Orphan Black series. In it, I give a slightly expanded explanation of genes and DNA.)
Background: The central dogma of molecular biology
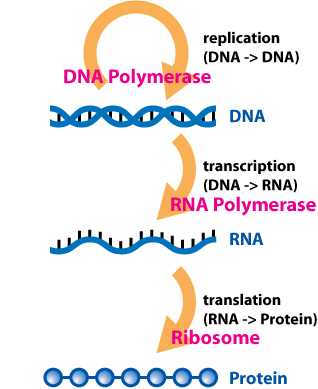
DNA has been called the blueprint of life for a very good reason. It seemingly contains all the information necessary to produce and maintain every earthly organism. The human genome is comprised of ludicrously long DNA molecules that are collectively comprised of over three billion smaller molecules, known as nucleotides, which we symbolize as A’s, C’s, G’s, and T’s. Each of these super long molecules is wound around proteins (histones specifically—more on them later) into one of 24 somewhat familiarly shaped chromosomes. (By the way, if your genome wasn’t wound onto histones and it was instead stretched into a straight line, it would be over 1.5 meters or 5 feet long.) Additionally, most chromosomes contain hundreds or thousands of genes.
But what’s a gene? A gene is a discrete string of consecutive A’s, C’s, G’s, and T’s that undergoes transcription, which is the mirror-image copying of that portion of DNA into a very similar molecule, called messenger RNA (mRNA). These mRNA molecules are then translated into yet another type of molecule, proteins, which do stuff and form structures in our bodies (not so coincidentally, proteins are also described in the earlier “the spindle protein” problem post; in biology everything is irritatingly, logically integrated). While DNA is great at holding information, it is not so great at anything else. The much shorter mRNA molecules (comprised of merely thousands of A’s, C’s, G’s, and U’s—and no that U is not a typo) are the intermediary between the inert genes locked in the DNA of our genomes and the proteins that make you a living, breathing being. In summary, a gene is a segment of DNA that is transcribed into a piece of mRNA that is translated into a protein; this, folks, is the Central Dogma of Molecular Biology.

For our purpose, I will add another layer of complexity—the phenotype, which is a rather fancy term for the observable physical characteristics of any organism. Typically, phenotypes can be caused by proteins, which are produced from mRNA, which is copied from DNA. Long ago, before anyone knew what genes were exactly, Brother Gregor Mendel (mentioned in last week’s post) had figured out that genes were passed from parent to offspring to produce discrete phenotypes or traits like the white versus red flowers or wrinkled versus smooth seeds of peas. Similarly, eye color and blood type are phenotypes in humans that are absolutely 100% governed by genes. In other words, your blood type (i.e., A, B, AB, or O) is determined by proteins that are produced from mRNA that is copied from DNA. As such, we generally consider genes to be comprised of DNA, but phenotypes, proteins, and mRNA are all consistent with genes.
So how is epigenetics “on top of” genetics? (or rather, what is epigenetics?)
The traditional paradigm presumed that phenotypes were mostly the product of genes and environmental conditions (i.e., nature and nurture—again, reviewed in a recent blog post). For example, if you have genes that would normally make you very tall, but you lacked proper nutrition as a child, you are likely to have stunted growth because of environmental effects, despite your height-increasing genes. There are many other well-documented environmental effects, ranging from acclimatization to altitude to pink coloration in flamingos. Until recently, genetic and environmental effects were thought to be all that determined phenotypes. Over the last 20 years, however, a number of unexpected observations have accumulated that have thrown this conclusion into disarray.
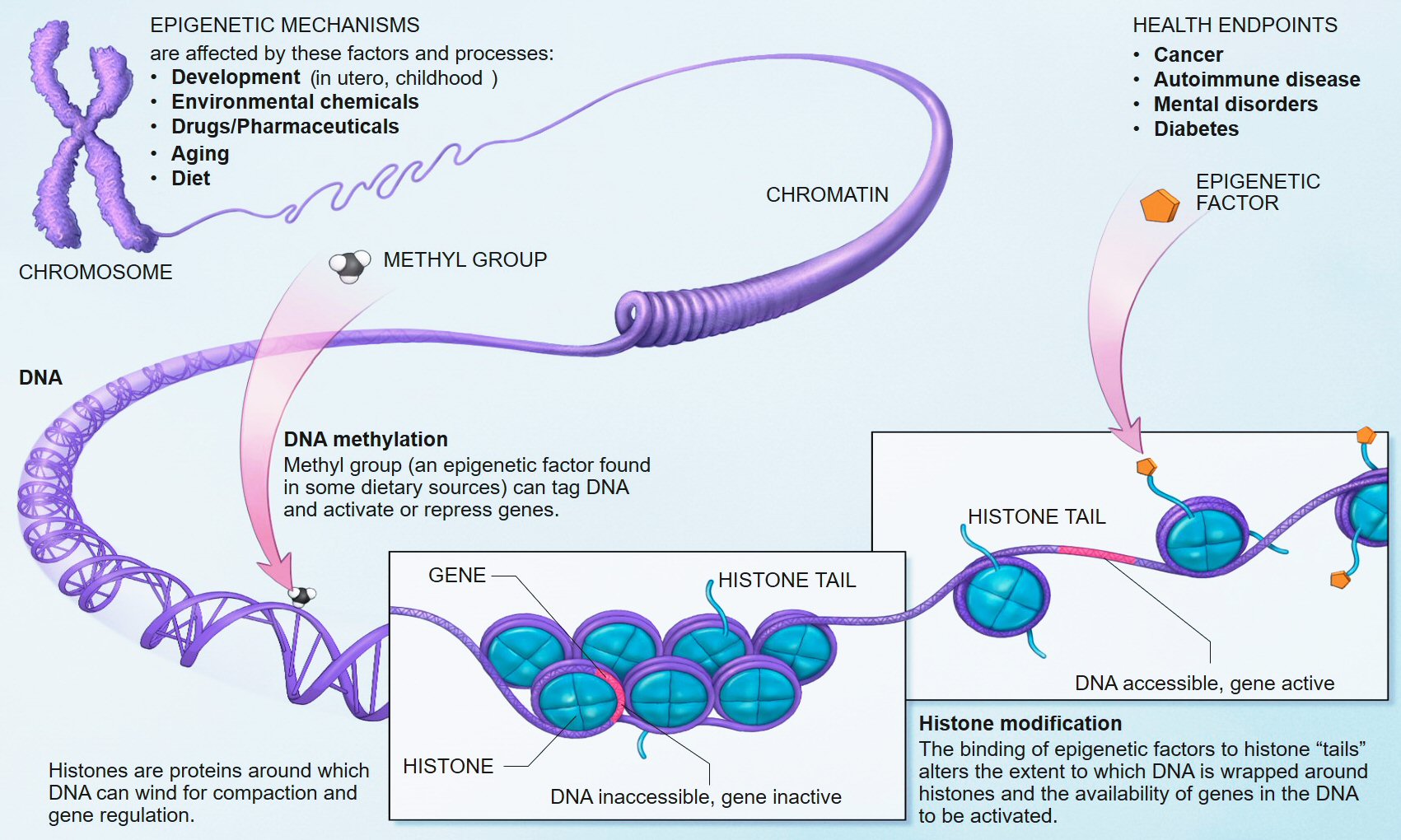
While the molecular mechanisms of epigenetics are still being figured out, most epigenetic changes appear to belong to two nonexclusive categories: modifications of how the DNA molecule is packaged into chromosomes and tiny additions to the individual nucleotides that make up the greater DNA molecules.
The first category of mechanisms is referred to as chromatin remodeling, generally by histone modification. Your genome is over 1.5 meters (5 feet) long, but the genome-containing nuclei of your cells are most certainly not. To fit your genome inside a cell, your DNA is wound up around proteins called histones, and these molecular spools are are bunched up together into classic X-shaped chromosomes during mitosis. Histones comprise a family of five nearly identical proteins that work in groups of eight to physically organize the genomes of nearly all eukaryotes—basically every organism except bacteria and the superficially similar archaea. These DNA-wrapped groups of eight histones are then neatly stacked together. Because of this histone-imposed structure, the DNA in your genome doesn’t knot like so many other strings, for example, the notorious earbud cords.
While this organization may improve the functioning of cells and the fidelity of mitosis, it can also impact the central dogma, specifically the transcription of DNA into RNA. Some parts of your DNA are tucked deep within these densely wrapped molecules, while others are totally exposed. Think of a spool of thread—some thread is visible, but most of it is under many, many layers.
However, these histones can be modified through post-translational processes to makes these protein spools bind DNA more or less tightly, thus impeding or facilitating the transcription of genes. After histone modification, some genes are much, much more easily copied from the exteriors of these spools while others are buried so deep that they will never be transcribed. In summary, (referring back to the central dogma) the way your chromosomes are wrapped around histones affects when and how much mRNA is transcribed from your DNA, which affects the production of your proteins, which may alter your phenotype.
The second category of epigenetic mechanisms includes tiny molecular additions to the DNA molecule that do not alter, add to, or remove the A’s, C’s, G’s, and T’s that comprise each gene. Specifically, A’s and (mostly) C’s are methylated; that is, these two nucleotides can pick up CH3+ ions, or rather, a methyl group. Though the specific mechanism is unclear, when a stretch of DNA contains methylated nucleotides, any genes it may contain are transcribed at a much lower rate, which decreases the amount of mRNA, which decreases the amount of protein, which may or may not alter your phenotype.
Additionally, there is one totally perverse aspect of epigenetic variation—it is somewhat heritable. That is, epigenetic variants such as chromatic modifications and methylated nucleotides may be inherited either by new daughter cells (through mitosis) or even passed on to offspring. Unless an enzyme comes along to reprogram epigenetic signals, they tend to transmitted to cells and offspring alike. For this reason, epigenetic variation may appear to violate a central tenet of evolution—that is, acquired traits may be passed on to future generations. For this reason, some evolutionary biologists have controversially invoked epigenetics as a mechanism for Lamarckism. I really wish I could expound on the views of Jean-Baptiste Lamarck, the French 18th century naturalist who contributed greatly, but incorrectly, to early thoughts on evolution, but I think that would take us even further from discussing Orphan Black, or even the modern science that is referenced on the show.
What is the biological function of epigenetics?

While histone modifications and DNA methylation might sound like molecular monkey wrenches that can only sabotage organisms and cause horrible diseases, they are actually perfectly normal parts of an organism’s inner working. One of the earliest and best characterized epigenetic effects is known as genomic imprinting or parent-of-origin effects. Within these effects, specific genes inherited from mothers are methylated, while other, totally different specific genes inherited from fathers are also methylated. Because of this differential methylation, either the maternal copy or paternal copy of a gene may be “turned off.”
As noted in my previous article on the spindle protein problem, the present impediment to successful reproductive cloning is replicating such methylation patterns in cloned embryos. Because the DNA within cloned embryos did not undergo the epigenetic reprograming that results from normal reproductive processes, the wrong genes are either turned off or on, and cloned embryos are ultimately inviable. Most of the epigenetic effects that occur within embryonic development are poorly understood, but they are absolutely fundamental to the development of mammalian embryos like those of primates. DNA methylation is also responsible for slowing the spread of transposable elements, a nasty genomic parasite that is contained in the DNA of most multicellular organisms. By “turning off” these genes, selfish genetic elements are kept from copying themselves and inserting in new places throughout genomes.
However, epigenetics has also been implicated in cancer. For example, the genes that encode tumor suppressors, a type of protein that normally limits uncontrolled cell growth, are often methylated in association with cancer. When tumor suppressor genes are “turned off” through epigenetic mechanisms, it is as though a cell does not have a working copy of those genes, and so cancerous growths can grow unabated.
I may seem to have gotten off topic, but the point is, epigenetics has a broad swath of effects. Asking about the effects of epigenetics, is a lot like asking about the effects of genetics. Epigenetics can theoretically influence any trait that is genetically controlled, even when there is no genetic variation for a trait, as one would suspect to be the case for Sarah, Cosima, Alison, et al.
Can epigenetics explain the differences among clones?

To answer that question, we must consider the ultimate causes of epigenetic variants. So far, we have only considered proximate causes of epigenetic variants—that is, the molecular mechanisms by which these weird, somewhat heritable effects are produced. But something must trigger these epigenetic changes, right?
We are currently in the midst of an epigenetics research boom. While the conclusions are often contradictory or confusing, a few general trends have emerged regarding the roles of in utero nutrition and stress in epigenetic programming. (Again, please remember my earlier warning—epigenetics research is in its infancy.)
When parents survive dire hunger, their offspring can receive epigenetic marks that alter gene expression. Stranger still, this effect can follow experiences of both mothers and fathers. The internal environment of the womb is vital to the proper development of offspring so epigenetic effects induced by moms’ diets are unsurprising. For example, epigenetic effects have been uncontroversially shown to result from inadequate prenatal consumption of folate (a B-vitamin); however, folate consumption by rodent fathers can influence epigenetic marks left on the paternal contribution to an offspring’s genome. (A broad review of the many epigenetic changes that can befall the offspring of poorly nourished parents is available here.)
While these explicitly nutritional studies are convincingly accumulating, there a small handful of epigenetic studies have been published that consider the effects of stress either in isolation or in combination with extreme hunger. One series of studies has made headlines again and again—the finding that the adult descendants of Holocaust survivors express higher levels of stress hormones than other Jewish adults (two quick reviews here and here). In other words, the authors of this study are suggesting that intense trauma, such as that endured by Holocaust survivors, can be transmitted across generations via epigenetics.
This research was obviously conducted in humans, which are notoriously and ethically difficult to manipulate. Accordingly, it's no suprise that the mechanism is still poorly defined. However, a related study was performed using mice that were scared before conception. (Yes, you read that right, scared.) In this study, mice were behaviorally conditioned to associate a smell with an electrical charge. The offspring of these mice were more likely to exhibit stress responses to the smell, even when they were not shocked themselves!
Again, many of these studies do not connect phenotypes with specific epigenetic marks, but they collectively suggest that many, many factors can influence phenotypes without altering the genes or environments of offspring. In other words, epigenetics is a mechanism that violates the central dogma of molecular biology, something most biologists thought was literally impossible just a few decades ago.
Accordingly, the personality differences among nearly genetically identical clones may have little to do with their formative childhood experiences and more to do with the procedures that created their zygotes and the conditions of their gestations inside the wombs of surrogate mothers. Perhaps the fertility of Sarah and Helena is a result of an important gene being unwittingly “turned on” by their birth mother. Maybe Alison’s penchant for organizing, taking charge, and other type-A-personality behaviors comes from a gene that was “turned off” by conditions within her birth (but nonbiological) mother. To speculate further, stress hormones expressed during the pregnancy that produced Alison may have predisposed Alison to think, feel, and act like her birth mother (perhaps drink like her as well). Heck, it is even possible that the Creutzfeldt–Jakob-like symptoms exhibited by the Castor clones stem from prions, which are actually an extremely rare type of epigenetic mechanism. But of course, I might be getting carried away here. Like any good scientists, we’ll need much more data (i.e., episodes of Orphan Black) to test this epigenetic hypothesis.