In the season 1 episode, CQB of The Expanse, the flagship of the Martian Congressional Republic Navy's Jupiter fleet, The MCRN Donnager, finds itself in a space battle with the six ships that attacked the Canterbury. Seriously outgunned, the Martian ship takes heavy damage from railgun fire; Canterbury med-tech, Shed Garvey (Paulo Costanzo) is struck in the head with a railgun round that punches several holes in the Donnager's hull.
Instead of exploding outward and sending the Canterbury crew into space, the small group must quickly work to seal the holes before everyone asphyxiates. The Earth's atmosphere contains approximately 21% oxygen and we can assume the same applies for spaceships in The Expanse. We lose consciousness when this falls below 10%. While there is no danger from an explosive decompression, as seen on a 2004 episode of the Mythbusters, the crew must still work quickly before their air runs out. Why don't we see everyone get sucked out like we see on just about every other movie (the two recent Star Trek movies are a good example), and can science tell us how long the crew has to patch the holes before everyone dies? Well, actually, it can.
The Best Kinetic Weapon ever is the Railgun!
The space battle between the Donnager and her unnamed assailants quickly moves from fighting with torpedoes to a Close Quarters Battle (CQB) using railguns. Railguns have the advantage in that they can quickly accelerate a projectile to far greater speeds than conventional weapons. In real-life, explosive-powered military guns cannot achieve a muzzle velocity more than 2 kilometers per second but railguns can easily exceed 3 km/s, thus providing greater range and more punch.
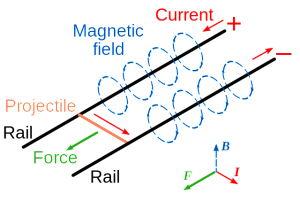
In principle, the physics behind the railgun is very simple. A large current passing through the two wires or rails generates a magnetic field; the larger the current the larger the magnetic field. The projectile is a conductor that connects the two rails. The current passes through the projectile it experiences a force known as the Lorentz Force. It is this force that accelerates the projectile to great speeds. This is also the same force that moves the armature in an electric motor which makes the physics behind the railgun is pretty much similar to an electric motor. Physics also shows that the larger the current, the larger the Lorentz Force. If you can store a lot of energy into a bank of capacitors and dump all that energy into your railgun, you have a pretty formidable weapon.
Railgun: Advantages and Disadvantages
Railguns have one advantage over cannons and explosive weapons. As long as a current flows through the circuit the projectile will continue to accelerate. Unlike a conventional weapon, the projectile is pushed by expanding gases. As the projectile moves along the chamber and the volume of the expanding gas increases, the force pushing on the projectile decreases. Railguns, unfortunately, require lots of power and the Donnager eventually has to divert power from propulsion to their weapons for CQB. This is not too far removed from reality. The US Navy's next generation all-electric battleship, the Zumwalt-class Destroyer, will be able to produce the currents needed to operate a railgun. To launch a projectile, power will be diverted from the engines to the gun turrets. Power can be redivered to the engines after the gun is finished firing.
During the battle, the Donnager is hit and the railgun projectile penetrates several walls including the room the crew of the Canterbury is located. We see that air starts leaking out the room and no one gets sucked out but this does not mean that everyone is not in danger. They must act quickly or everyone will die. But how long do they have? It turns out physics again has an answer.
The Physics of Decompression and Constricted Airflows
We do not see the room explosively decompress when the railgun projectile shoots through the Donnager's hull and wall. Except for the fact that air is being sucked out into "hard vacuum," everyone manages to stay in their seats. This happens for a few reasons. The first is the hole, or constriction, is too small for all the air in the room to explosively leave the room. The second deals with the fact that air is made of atoms. Air escaping the hole in the hull to the vacuum of space leaves at approximately the speed of sound. As air molecules exit the hole, the remaining molecules have to "catch up." Think what happens in a traffic jam. All cars do not move together. One car slowly inches forward and then everyone follows. This means there is no explosive decompression unless the entire wall is suddenly removed. While the crew has some time to act, that time is very limited.
Scientists and engineers have looked at the physics of constricted airflow for some time with regard to aircraft. It is a very good idea to know what happens to an aircraft if a hole forms while in flight. A. Fliegner was one of the first engineers to look at this problem and was able to work out how much air leaves depending on the pressure inside a cabin and the size of a hole. We know this as Fliegner's Formula:

where
 is the mass flow,
is the mass flow,  is the area of the hole,
is the area of the hole,  is the pressure inside the cabin, and
is the pressure inside the cabin, and  is the room temperature.
is the room temperature.
As we expect, the air flow depends on the hole's area, cabin pressure and temperature. Of course, Fliegner's Formula is not that accurate. As the leak progresses, the pressure in the cabin drops and this also affects air flow through the hole. Have no fear, we can use the equation and a little physics to figure out the time it takes the pressure to drop to a certain level. We get:

We have some new variables:
 is the volume of the room they are in and
is the volume of the room they are in and  is the final pressure. Now that we have figured out the equation, we can model what happens inside the cabin and how much time the Canterbury crew have to act.
is the final pressure. Now that we have figured out the equation, we can model what happens inside the cabin and how much time the Canterbury crew have to act.
The Human Race needs to breathe to survive
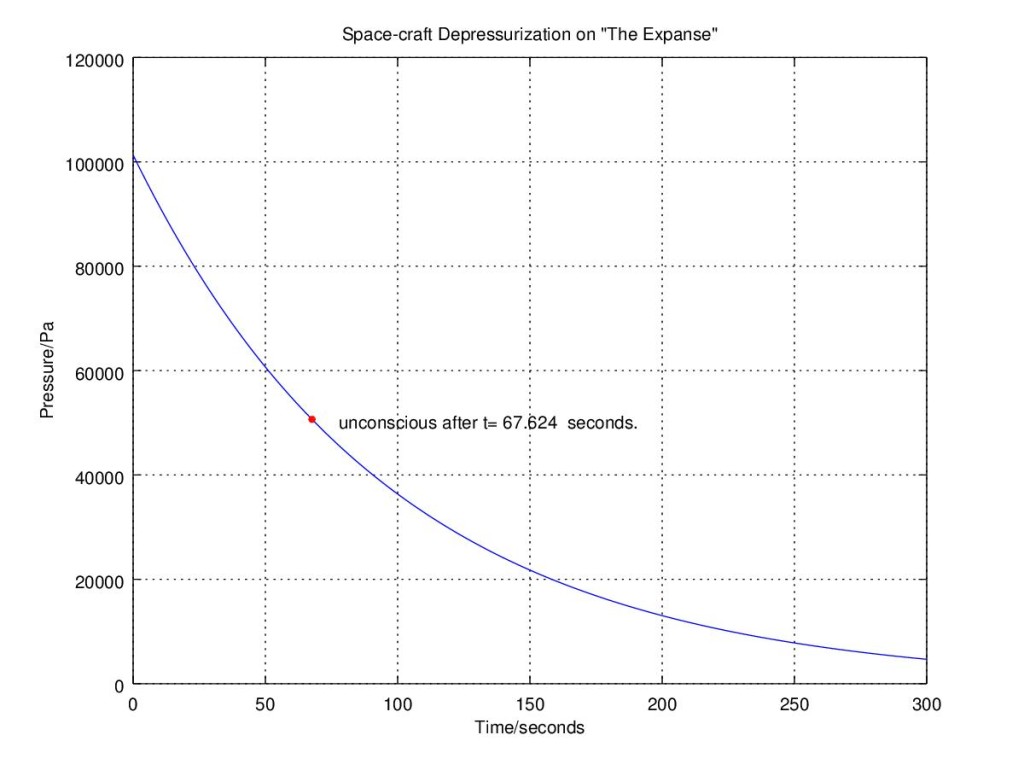
Air is approximately 20% oxygen. If that level falls to approximately 10% or half atmospheric pressure, you will not have enough oxygen to function and become hypoxic. While you would not necessarily die, you can fall unconscious. We assume that the Canterbury crew can not help themselves and will eventually die as the cabin pressure decreases until all the air is sucked out to the vacuum. Basically, everyone dies when the pressure falls to 50%. Maybe Sed is the lucky one here.
While we do not have the exact dimensions of the room, we can make a few assumptions. Based on the body sizes of the crew, I assume the room is 10 meters by 10 meters by 5 meters or 500 cubic meters in size. The temperature of the room is about 27℃ (80℉ or 293K). If we plot the graph over time we see that the pressure drops to half its value where everyone has a little over a minute to plug up the holes.
Does "The Expanse" get it right?
Assuming that everything happens in real-time, from the moment Sed loses his head to the second the holes are sealed, the crew manages to do seal the holes with some seconds to spare. While the estimated size of the room may be larger than it really is, the point is... They survive! The show definitely gets the science right and the urgency the crew must act to save their lives.
Also published on Medium.